
Property |
Meaning |
Importance of Property |
1. Transparency |
Light passes thru water. |
Light reaches chloroplasts in cells & aquatic plants. |
2. Universal Solvent |
Many compounds dissolve in water. |
Dissolved compounds can be brought to cells (via sap or blood) or move about cell cytoplasm. |
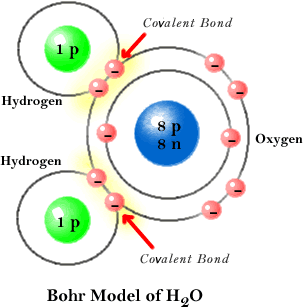
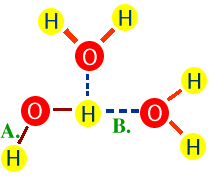
3. Cohesion4. Adhesion |
Water molecules stick together due to H bonds.Water molecules stick to other molecules. |
Small animals may walk on water.Capillary action. Water pulled to top of trees. |
5. Heat Capacity |
Large amounts of energy are needed to raise temp of water. |
Water bodies have stable temperatures. Body temps can be maintained. Transfer of heat from warm to cool body parts. |
6. High boiling point.7. Density of Ice |
Much energy needed to pull water molecules apart.Ice is less dense than water so it floats. |
In nature, water rarely boils so life is spared.Ice insulates organisms living beneath. |
8. Evaporation |
Evaporation (boiling) requires much energy. |
Evaporation can cool warm cells. |

Solvency of Water (how water dissolves ionic compounds) - Northland University
Hydrogen Bonds - Northland University
Polarity of Water Molecules: Formation of Ice - UC Davis