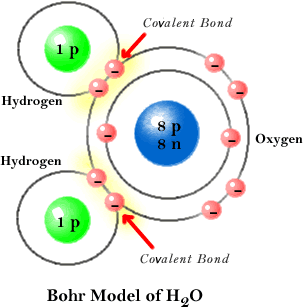


The diagram above shows the orientation of the 2 hydrogen atoms and the 2 other pairs of electrons around the central oxygen atom within a water molecule. Each gray spoke radiating out from the oxygen represents a pair of electrons, so oxygen has filled its outer shell by sharing electrons with the 2 hydrogens.
The important factor shown by this diagram is the angle between the 2 hydrogen atoms. This angle is also the same between the hydrogen atom and the pairs of electrons shown at lower right. The significance of having 2 hydrogen atoms on one side of a water molecule is that the oxygen, being a stronger electron grabber than hydrogen, is able to pull the electrons shared with each hydrogen towards it. The result is an unequal sharing of the electrons.
This makes a water molecule polar, in that the oxygen end of the molecule has more electrons (a negative charge), while the hydrogen end has a slightly more positive end (as the electrons are found there less frequently). Having both a positive and negative end, water thus acts like an electromagnet. The positive end is able to attract negative ions or the negative end of other polar molecules. The negative end is able to attract postive ions or the positive end of other polar molecules. Because water does this very well, it is able to dissolve many substances, and it is thus called a universal solvent.