
Water is a polar molecule, in that the oxygen end of the molecule has more electrons (a negative charge), while the hydrogen end has a slightly more positive end (as the electrons are found there less frequently). Having both a positive and negative end, water thus acts like an electromagnet. The positive end is able to attract negative ions or the negative end of other polar molecules. The negative end is able to attract postive ions or the positive end of other polar molecules. Because water does this very well, it is able to dissolve many substances, and it is thus called a universal solvent.
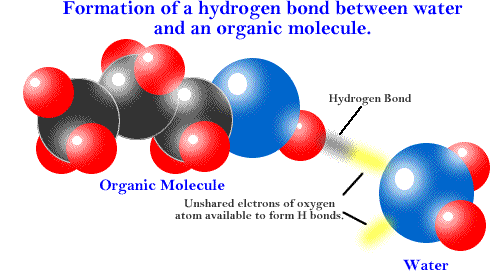
Many other molecules form hydrogen bonds. Hydrogen bonds between various atoms in a protein help give it its 3-dimensional shape.