
1) Globular proteins with specialized 3-D shapes
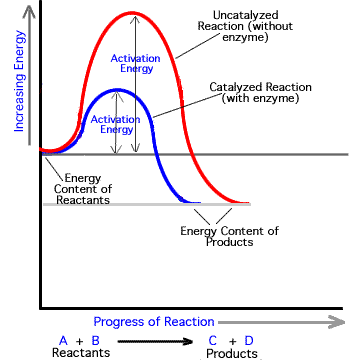
2) Lower activation energy by
a) bringing two substrates together (greater chance to react)
b) stress bonds of a substrate (to break them)
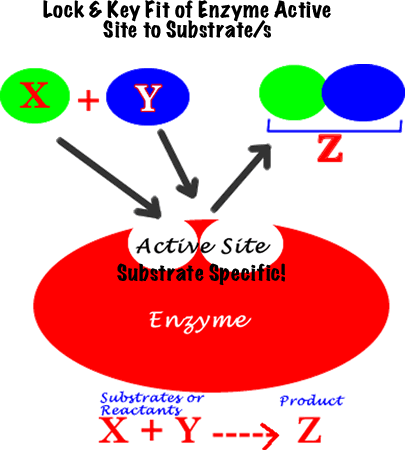
Induced fit: enzyme changes shape of active site so substrate fits perfectly. This allows some enzymes to catalyze several different reactions.

Note: The scientific name of all enzymes ends in -ase.
a) Temperature: Disrupts hydrogen bonds, alters protein shape (denature)
b) pH: hydrogen ion concentration disrupts bonds between amino acids
c). Substrate Concentration: Increased substrate concentration increases reaction rate until all enzymes are involved, then reactions level out
d) Enzyme Concentration: Increased enzyme concentration increases reaction rate until all substrate is used up, then reactions decrease.
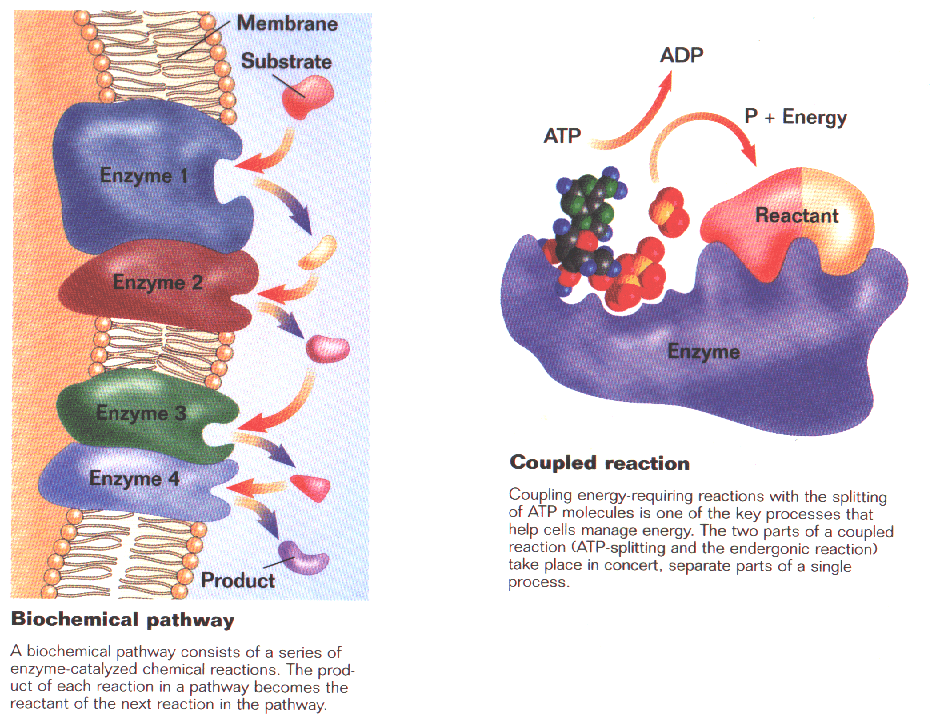
Several to many enzymes are placed side by side on membranes within cells, each enzyme completing one of the many steps to convert 1 substance into another. ("Assembly Line")

Amino Acids, Proteins, Dehydration Synthesis & Protein Shapes
Dehydration Synthesis & Hydrolysis (RM Chute) (See examples concerning proteins & carbos at bottom of that page too!)
Denaturation of a Protein (Cooking an Egg)
Factors Affecting Enzyme Activity
Enzymes (Prentice Hall) - Comprehensive review of enzymes. Click on Web Tutorial 6.2, then on Animation.
How Do Enzymes Work? (McGraw Hill)
Enzymes (Click all but the Allosteric Enzymes link!)
How Do Enzymes Work? (Starch Digestion)
How Temperature Affects Molecular Motion (BEC)
Noncompetitive Enzyme Inhibition (Allosteric Enzymes)
Biochemical Pathways (Several different enzymes in close proximity that help change a substrate via several steps into its product)
a) [Competitive inhibitors]: Competitor molecule binds at active site, prevents substrate from binding.
b) [Non-competitive inhibitors]: End product binds at a allosteric (different site), changes shape of active site. Substrate can't bind.
c) Allosteric site: region where non-competitive inhibitor binds changing shape of enzyme.