DEHYDRATION & HYDROLYSIS REACTIONS
Dehydration reactions involve monomer
molecules (like amino acids or simple sugars) bonding to each other. When they
bond, an OH and an H (H2O) are removed from them. Because the newly bonded molecule
loses water, this is known as dehydration. The upper diagram illustrates dehydration,
where the green-shaded circles represent individual monomers.
Hydrolysis reactions involve breaking
polymer molecules such as polypeptides (large proteins) or starches into their
monomer forms (amino acids and glucose molecules respectively). This can only
be done when water is added to each of the bonds between the monmer molecules
(shown as green shaded circles below). The second diagram below illustrates
the process of hydrolysis, where water is added to break the big molecule into
smaller components.

Dehydration Reactions (Building of larger molecules) add energy
to organic molecules.
Hydrolysis Reactions (those that tear apart molecules) release
energy from the molecules.
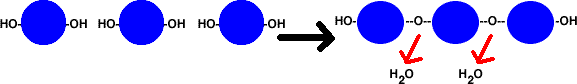
An example of a dehydration reaction.
Slichter


