[Sophomore Biology: Enzyme Lab Menu]
Energy & Chemical Reactions In Cells
Read Pages 77 - 81
Reactants (Ingredients) ----------------> Products
(ending materials)
2H2O2 ---------------------> 2 H2O + O2
C6H12O6 + O2 --------------------> CO2 + H2O + energy to make ATP
All reactions need energy to start them.
Activation Energy: Energy needed to start a
chemical reaction.
Wood, gas, etc. need lots of energy to start the "burning" reaction with
O2 . (much heat, light, electricity, etc..) Cells use enzymes to begin most
reactions (such as "burning" of glucose & O2).
Enzymes: Proteins that lower the amount of energy
needed for reactions to occur. They put the reactants together so they occur
easily! They are a type of catalyst (any substance that makes reactions occur
easily (like lead in gas)).
Substrate: The reactant(s) that the enzyme binds
to. Enzyme & substrate bind like lock & key.
Each enzyme helps only 1 chemical reaction due to lock & key fit on substrate/s.
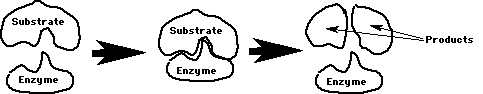
Notice the active site on the enzyme above, where the substrate (reactants) fit to the enzyme in a "lock and key" effect.
The enzyme is not changed by the reaction. It finds another substrate to
continue the reaction. Review Figures 5-1, 5-2, 5-3, & 5-5 in your book. Review
Questions 1-4, page 81 in your book!
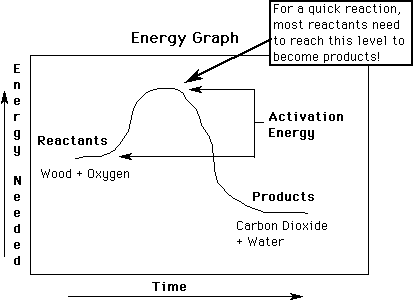
The graph above shows energy during a chemical reaction. Traveling up
the Y- axis means there is more energy. The graph shows that the reactants
(wood and oxygen) have more energy than the products (CO2 and H2O). Wood
and oxygen both must gain energy (this is called activation energy) before
the reaction can begin to make the products. This is why energy from a
match is needed to start the fire.
Slichter


