2H2O2 ---------------------> 2 H2O + O2
C6H12O6 + O2 --------------------> CO2 + H2O + energy to make ATP
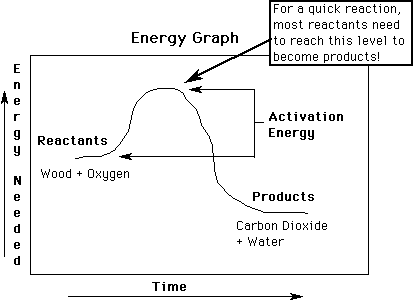
Activation Energy: Energy needed to start a chemical reaction.
Wood, gas, etc. need lots of energy to start the "burning" reaction with O2 . (much heat, light, electricity, etc..) Cells use enzymes to begin most reactions (such as "burning" of glucose & O2).
Enzymes: Proteins that lower the amount of energy needed for reactions to occur. They put the reactants together so they occur easily! They are a type of catalyst (any substance that makes reactions occur easily (like lead in gas)).
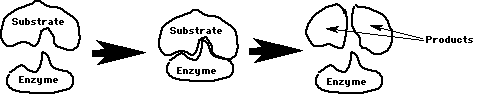
Substrate: The reactant(s) that the enzyme binds to. Lock & key fit.
Each enzyme helps only 1 chemical reaction due to lock & key fit on substrate/s.

The enzyme is not changed by the reaction. It finds another substrate to continue the reaction. Review Figures 5-1, 5-2, 5-3, & 5-5 in your book. Review Questions 1-4, page 81 in your book!