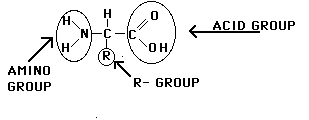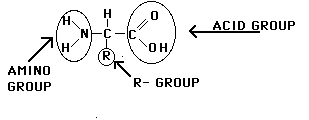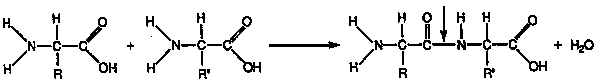CHEMICALS OF LIFE:
1. WATER
2. CARBON BASED (ORGANIC) COMPOUNDS
IMPORTANT PROPERTIES of WATER:
1. UNIVERSAL SOLVENT.- water dissolves many substances
2. SURFACE TENSION
A. COHESIVE: water molecules show attractions to each other.
B. ADHESIVE: water molecules show attractions to other substances.
3. HIGH BOILING POINT: Good insulator-keeps body temp same as
much energy needed to raise temp 1o degree Celsius.
4. GOOD COOLANT: Evaporation takes energy from skin, cooling
it.
5. SOLID FORM (ICE) IS LESS DENSE THAN LIQUID FORM, SO ICE FLOATS.
THE ICE CAN THEN ACT AS AN INSULATOR.
6. pH: dissociation (breaking into ions) of H2O into H+ and OH-
H2O ----> H+ + OH-
A. ACID: more H+ ions (pH 0-7)
B. BASE: more OH- ions (pH 7-14)
C. NEUTRAL: H+ = OH- (pH =7)
D. BODY FLUIDS: pH = 7.4
ORGANIC MOLECULES
Carbon atoms bonded to at least 1 hydrogen atom. Frequently
bonded to oxygen or other carbon atoms. Carbon is a unique element due to its
ability to form covalent bonds that are strong and stable.
MONOMERS: Small organic molecules.
POLYMERS: Macromolecules (macro
= large). Several monomers are bonded together to make larger polymers.
TYPES OF ORGANIC COMPOUNDS:
A. Proteins
B. Carbohydrates
C. Lipids
D. Nucleic Acids
PROTEINS
Large molecules that make up structures in cells. >50% of dry
body wt..
Other importances: Enzymes, hormones, contractile fibers of muscles,
O2 transport, toxins, etc.
1. MONOMER PROTEIN FORMS: AMINO ACIDS: Building blocks of all
proteins. 20 types. All contain carboxyl group(COOH), amino group (NH2), & R
group (variable). R group is the only difference for each a.a..
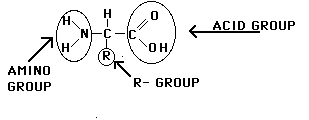
1. POLYMER FORMS: Called polypeptides (2 or more a.a.s bonded
together). Peptide bond connects 2 a.a.s together. Peptide bond occurs between
carboxyl group of one a.a. & amino group of 2nd a.a..
DEHYDRATION: Reaction to form a peptide bond, where H2O is released
as bond forms.
aa + aa ----> protein + H20
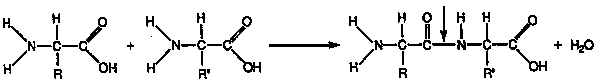
DIPEPTIDE: 2 a.a. s linked together.
PROTEIN: Composed of one or more polypeptide chains linked together.
PROTEIN STRUCTURE: A protein's 3-D shape is dictated by its a.a.
sequence. One change in a.a. sequence may change the whole structure of a protein
as well as its function.
ENZYMES
ENZYME: Protein that acts as a catalyst. They control the reaction
rates of most if not all reactions in an organism. All end in "ase" (Ex.- Catalase,
lactase, amylase, etc.).
CATALYST: Any chemical that increases the rate of a chemical
reaction. The catalyst is not changed or used up. Not all catalysts are enzymes.
SUBSTRATES: The reactants of an enzyme catalyzed rxn.
PRODUCTS: What the reaction makes.
ACTIVATION ENERGY: Amount of energy needed to raise substrate
molecules to the transition state at which the reaction can occur. Enzymes lower
the activation energy of reactions.
ACTIVE SITE: A small part of the enzyme is shaped such that the
substrate/s can fit to it and thus cause the the reaction to occur more easily.
Slichter