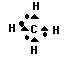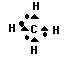4. The type of bonds shown in the molecule below are covalent
bonds, where the hydrogen atoms each share an electron with the electrons
of carbon. The result is that all 4 hydrogen atoms gain full outer energy levels
(with 2 electrons) while the carbon atom gains 4 more electrons via the sharing
to have a full outer energy level (8 electrons).
 ----- -----
----- ----- 
Slichter