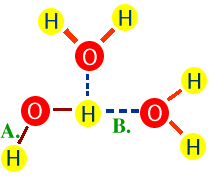
26. Water is considered a polar molecule because the
oxygen atoms doesn't equally share the electrons with the hydrogen atoms. The
result is that the hydrogen atoms develop a slight positive charge while the
oxygen atom gains a slight negative charge. Due to these slight charges, the
hydrogen atoms of one water molecule form weak hydrogen bonds with the oxygen
atoms other water molecules.
 ----- -----
----- ----- 
Slichter

