How pH Affects Reation Rate
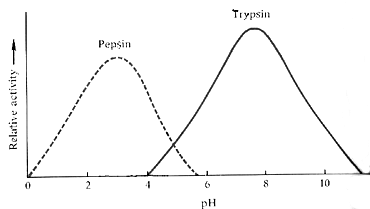
The graph shows that when the pH is changed the reaction rate
of the enzyme changes too. More specifically, if we use Trypsin from the graph
above as our example, at a pH of 4, the reaction rate is zero. As the pH increases
towards the 8, the reaction rate increases until near a pH of 8, it peaks. Above
that peak, the reaction rate begins to decrease towards zero again as the pH
nears 11.
What happens can be explained in terms of the shape and structure
of the enzyme molecule. As a type of protein, enzymes are easily affected by
changes in pH. At their optimum pH (in the case of Trypsin, this is slightly
below a pH of 8), the shape of the enzyme is such that the active site can fit
perfectly with the substrate. As the pH decreases from, or increases from the
optimum, the acid or base conditions begin to disrupt some of the hydrogen bonds
between loops of the protein chains. If the disruption occurs at or near the
active site, the active site becomes distorted and substrate can not fit perfectly.
Thus not all enzymes in the solution will be able to catalyze their reaction.
With increasing or decreasing pH, more enzymes become denatured, and fewer enzymes
are able to form that enzyme-substrate complex. The reaction rate continues
to decrease. At somepoint, all the enzymes are denatured, and the reaction rate
falls to zero. For Trypsin, this occurs near a pH of 4 and again near a pH of
11.
Links for Further Research
Slichter

