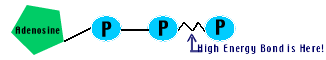

ATP: Adenosine TriPhosphate - Energy carrying molecule. Carries measured doses of energy. Used to facillitate many cell reactions.
![]()
Oxidation: Loses e- & energy. (Gains Oxygen.)
Reduction: Gains e- & energy. (Loses Oxygen.)
"Oxygen is Loss (of e-), Reduction is Gain (of e-)"
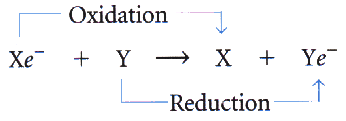
Comparing Oxidation & Reduction Reactions
|
Oxidation Reactions
|
Reduction Reactions
|
| Adding oxygen atoms to a substance. | Removing oxygen atoms from a substance. |
| Loss of electrons from a substance. | Adding electrons to a substance. |
| Loss of hydrogen atoms from a substance. | Addition of hydrogen atoms to a substance. |
| The substance loses energy when it loses electrons & hydrogen atoms. | The substance gaining electrons or hydrogen atoms gains energy. |
As a result of oxidation reactions in living cells, hydrogen atoms tend to carry electrons and energy to the substance to be reduced.
Example #1:

Reduction: Carbon dioxide gains electrons and hydrogen to become glucose. It is reduced.
Oxidation: Water loses electrons and loses hydrogens to become oxygen. It is oxidized.