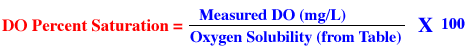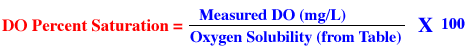An Introduction to Dissolved Oxygen
Oxygen is as important to life in water as it is to life on land. Most aquatic
plants and animals require oxygen for survival and the availability of oxygen
affects their growth and development. The amount of oxygen found in water is
called the dissolved oxygen concentration (DO). DO is a very important measure
of the health of a stream--the presence of oxygen in water is a positive sign,
the absence of oxygen in the water is often a sign that the stream is polluted.
Dissolved oxygen levels vary from stream to stream, as do organisms' tolerance
for dissolved oxygen levels. Factors which affect Dissolved Oxygen (DO): 1.
Temperature: Oxygen, like all gases, is more soluble at lower temperatures,
so generally the colder the water, the more oxygen it can hold!
2. Altitude/ Atmospheric Pressure: The higher the air pressure, the more
oxygen that water can hold. Water at sea level can hold more oxygen than water
found in a lake in the Cascade Mts. You can see this by pouring a cold soda
pop into an open glass. If you let the soda pop sit on the table, you begin
to see bubbles leave the pop and enter the air because there is less pressure
to keep it in the water. Eventually, the soda pop goes flat.....in other words,
it has lost all of its carbonation (carbon dioxide).
3. Water Turbulance:
4. The growth of plants: As plants are exposed to sunlight, they perform
photosynthesis, which converts carbon dioxide and water into sugar and oxygen.
The oxygen is released directly into the water, which raises the amount of oxygen
in the water.
5. The amounts of decaying organic materials (dead things) in the water:
The bacteria, molds, and other critters that decompose dead and decaying materials
use oxygen to break the dead things down into simpler things. As they use this
oxygen, the oxygen levels in the water decrease.
6. Physical, chemical, and biochemical activities in the water:
For short periods of time water may become supersaturate, holding more oxygen
or other gases than it is supposed to. Supersaturation can also be harmful to
aquatic organisms, causing a condition called gas bubble disease, which is similar
to the bends disease that deep sea divers may get if they surface too fast.
For meaningful interpretation of dissolved oxygen levels two pieces of information
are required, the amount of oxygen dissolved in the water sample, measured in
mg/L, and the temperature of the stream at the time DO was measured. With these,
the perecent saturation of the oxygen in the water can be determined. Other
factors, such as biochemical oxygen demand (BOD) affect the interpretation of
percent saturation levels, but for general purposes, the following guidelines
may be useful:
125% or more Supersaturation at levels dangerous to fish
90-100% Excellent conditions
80-90% Adequate conditions
60-79% Acceptable conditions
Below 60% Poor conditions. Water may be too warm or have high BOD levels
If oxygen levels in the water are to low, then aquatic organisms have difficulty
respiring (breathing). Some organisms have adapted to low oxygen in water (like
carp) or are able to ingest air directly (like bettas).
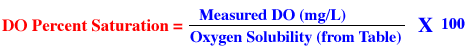
First the current barometric pressure and mean water temperature (ūC) are
compared to a special table (available from your instructor) to find the solubility
of oxygen in water at that air pressure and temperature. The mean of the sampled
Dissolved Oxygen as measured in mg/L is then divided by the oxygen solubility
and multiplied by 100 to get an answer which is in percent.
In rivers there is usually adequate mixing of water from the surface to the
river bottom. However, in very slow moving or very deep rivers there may be
little mixing of the water. This could cause differences in DO measurements
from the surface to the river bottom. This is usually the worst in the hot summer,
where rivers are shallow, slow moving and warm.
General Water Quality Standards for Oxygen
Type of Water Standard (mg/L DO)
Trout & Salmon Spawning 11.0
Cold Water fisheries 8.0
Cool Water fisheries 6.5
Warm Water fisheries 5.5
Reasons why water may be low in oxygen:
1. Rapid decomposition of organic materials, including dead algae, shoreline
vegetation, manure or wastewater sources, all decrease oxygen levels.
2. High ammonia concentrations in the stream use up oxygen in the process
of oxidizing ammonia (NH4) to nitrate (NO3).
3. At higher temperatures, less oxygen can dissolve in water.
4. Lack of turbulence (too many pools, not enough riffles) or mixing to expose
water to atmospheric oxygen results in low dissolved oxygen concentrations in
the stream.